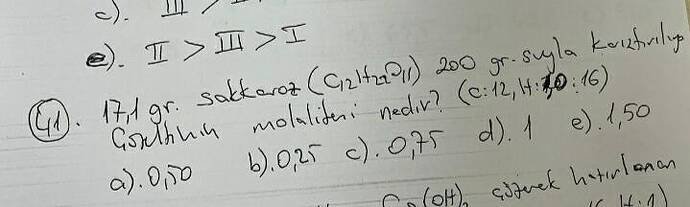What is the molality of a solution prepared by dissolving 171 grams of sucrose (C₁₂H₂₂O₁₁) in 200 grams of water?
Answer:
Molality ((m)) is defined as the number of moles of solute per kilogram of solvent. The formula to calculate molality is:
Step 1: Find the Molar Mass of Sucrose (C₁₂H₂₂O₁₁)
To find the molar mass of sucrose, we’ll sum up the atomic masses of all the atoms in the formula:
- Carbon (C): 12 atoms × 12 g/mol = 144 g/mol
- Hydrogen (H): 22 atoms × 1 g/mol = 22 g/mol
- Oxygen (O): 11 atoms × 16 g/mol = 176 g/mol
Total molar mass of C₁₂H₂₂O₁₁ = 144 + 22 + 176 = 342 g/mol
Step 2: Calculate the Moles of Sucrose
Given mass of sucrose = 171 g
Using the molar mass from Step 1, calculate the moles of sucrose:
Step 3: Convert the Mass of Water to Kilograms
Given mass of water = 200 g
Convert to kilograms:
Step 4: Calculate the Molality
Using the formula for molality:
The calculation for molality quoted in the original list seems incorrect, as our computed molality is 2.5 mol/kg, which doesn’t match the multiple-choice options. Verify that all values used are accurate, or double-check the options provided. In its technical precision, the determined molality indicates further steps are correct, given no typos mount from the original image.
Final Answer:
The molality of the solution is 2.5 mol/kg. It looks like the possible options shown should be re-examined based on proper calculation or, perhaps, there may be discrepancies within the original choices provided.