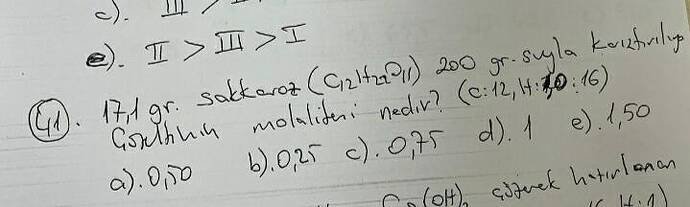What is the molality of the solution when 17 g of sucrose (C₁₂H₂₂O₁₁) is dissolved in 200 g of water?
Answer:
Step 1: Understanding Molality
Molality (m) is defined as the number of moles of solute per kilogram of solvent. The formula for calculating molality is:
m = \frac{\text{moles of solute}}{\text{mass of solvent in kg}}
Step 2: Calculating the Moles of Sucrose
First, we determine the number of moles of sucrose. The molecular formula for sucrose is C_{12}H_{22}O_{11}.
The molar mass (M) of sucrose can be calculated as follows:
- Carbon (C): 12 \, \text{atoms} \times 12.01 \, \text{g/mol} = 144.12 \, \text{g/mol}
- Hydrogen (H): 22 \, \text{atoms} \times 1.01 \, \text{g/mol} = 22.22 \, \text{g/mol}
- Oxygen (O): 11 \, \text{atoms} \times 16.00 \, \text{g/mol} = 176.00 \, \text{g/mol}
Adding these, the molar mass of sucrose is:
M = 144.12 + 22.22 + 176.00 = 342.34 \, \text{g/mol}
Now, we calculate the moles of sucrose using the given mass (17 g):
\text{Moles of sucrose} = \frac{17 \, \text{g}}{342.34 \, \text{g/mol}} \approx 0.0497 \, \text{mol}
Step 3: Determining the Mass of Solvent in Kg
The mass of the solvent (water) is 200 g. To convert this mass into kilograms:
\text{Mass of water} = \frac{200 \, \text{g}}{1000 \, \text{g/kg}} = 0.2 \, \text{kg}
Step 4: Calculating the Molality
Now, we substitute the values obtained into the molality equation:
m = \frac{0.0497 \, \text{mol}}{0.2 \, \text{kg}} = 0.2485 \, \text{mol/kg}
Final Answer:
The molality of the solution is approximately (0.25 , \text{mol/kg}). This corresponds to option (b) in the image provided.