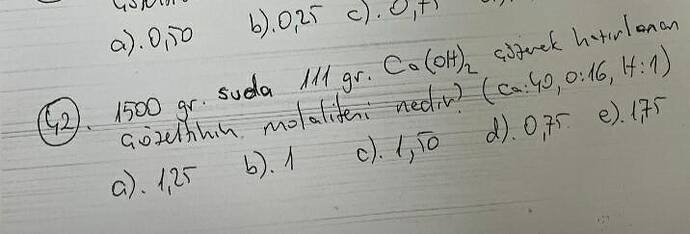What is the molality of a solution prepared by dissolving 111 grams of Ca(OH)₂ in 1500 grams of water?
Answer:
To find the molality of the solution, follow these steps:
Step 1: Calculate Moles of Ca(OH)₂
First, determine the molar mass of calcium hydroxide, Ca(OH)₂:
- Ca: 40.08 g/mol
- O: 16.00 g/mol
- H: 1.01 g/mol
Calculation:
\text{Molar mass of Ca(OH)}_2 = 40.08 + (16.00 \times 2) + (1.01 \times 2) = 74.10 \, \text{g/mol}
Now, calculate the moles of Ca(OH)₂:
\text{Moles of Ca(OH)}_2 = \frac{111 \, \text{g}}{74.10 \, \text{g/mol}} \approx 1.497 \, \text{mol}
Step 2: Convert Water Mass to Kilograms
The mass of water given is 1500 grams. Convert it to kilograms:
1500 \, \text{g} = 1.5 \, \text{kg}
Step 3: Calculate Molality
Molality is calculated using the formula:
\text{Molality} = \frac{\text{moles of solute}}{\text{mass of solvent in kg}}
Substitute the found values:
\text{Molality} = \frac{1.497 \, \text{mol}}{1.5 \, \text{kg}} \approx 0.998 \, \text{mol/kg}
Final Answer:
The molality of the solution is approximately 1 (b. 1).